Our Mission
Our long term goal is to determine how allergens and epithelial damage exhaust repair mechanisms in the airway mucosa, disrupting normal tissue healing and promoting the transition to epithelial and olfactory dysfunction.
We are defining the epithelial cell pathways triggered by native allergens and the downstream activation of resident immune cells, and examining how these two events drive the transition of the immune response towards allergic inflammation.
To understand these mechanisms, our lab has taken a multi-pronged approach:
- Identification of signaling pathways in an ex vivo system of allergen-triggered epithelial cell activation
- Identification of activation events in resident immune and epithelial cells in situ
- Defining the transcriptional profile of airway tuft cells in mice and in humans
Clinical study: Mediators of post-COVID anosmia
We recently began a clinical study to investigate the causes of decreased sense of smell (anosmia or hyposmia), which is associated with irritation of nasal and sinus mucous membranes. Sudden, pronounced loss of smell and/or taste is one of the core symptoms of COVID-19, and is often more severe and long-lasting than anosmia caused by other respiratory infections. We hope to learn more about how respiratory infections cause anosmia, and why some people who experience COVID-19-related anosmia eventually regain their sense of smell and/or taste while others do not.
Tuft Cells: Nasal Danger Sensors
Allergen-triggered epithelial cell activation
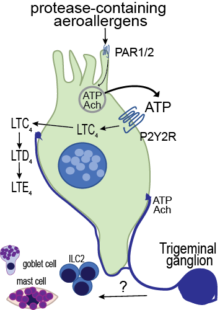
Our research focuses on a specialized epithelial cell called the airway tuft cell (or brush cell). The tuft cell is enriched for G-protein-coupled receptors (GPCRs), senses allergens in the airway lumen, engages ATP feed-forward loops to enhance mediator release, and is highly innervated in certain areas of the respiratory mucosa. Ultimately, these features allow for fast propagation of activating signals throughout the airway mucosa (Ualiyeva Sci Immunol 2020).
Discover more
Eicosanoids as Innate Effector Molecules
Cysteinyl leukotrienes (CysLTs) are potent eicosanoid mediators of bronchoconstriction, vasodilatation, and type 2 inflammation classically associated with established allergic inflammation, where they are derived from sensitized mast cells, eosinophils, and macrophages, and hence termed “leuko” trienes. Recently, we reported tuft cell generation of cysteinyl leukotrienes in the naïve nasal mucosa, identifying a role for these lipid mediators in the primary innate mucosal response to aeroallergens (Ualiyeva Sci Immunol 2020). Cysteinyl leukotrienes are required for the lower airway inflammation triggered by allergens (Bankova Sci Immunol 2018), establishing their role as initiators of allergic inflammation.
Linking Tuft Cells to Mast Cell Activation
In initial studies with Alternaria, we found that mast cells in the nasal submucosa are activated in an IgE-independent fashion. A possible link between tuft cell-derived cysteinyl leukotrienes and mast cell degranulation through a sensory nerve activation mechanism was not explored. Building on our new findings of LTC4 generation by brush cells located in close proximity to sensory nerve fibers and data from several groups demonstrating that the second cysteinyl leukotriene receptor in highly expressed in airway innervating sensory neurons, we will determine if sensory nerve activation through brush cell-derived LTC4 is engaged in the protective airway responses of mucin release and in propagation through mast cell degranulation.
Cover Story: Mouse mast cell proteases 4 and 5 mediate epidermal injury through disruption of tight junctions
Thermal stress injury to the skin induces mast cell degranulation within a minute. Heat-induced tissue mast cell degranulation is reversible when the ambient temperature is decreased under 4°C. Tight junctions in the epidermis are disrupted in a mast cell-protease dependent fashion but desmosomes are preserved. Using mice with targeted disruptions of mouse mast cell proteases (mMCP), we identified claudin 4 as the molecular target of mMCP 4 and confirmed the specificity of this effect as it is absent in mice lacking mMCP5.