NEW PUBLICATION
Click to enlarge the graphical abstract!
Minichetti D.G. & Boyd A. et al., Journal of Allergy and Clinical Immunology, 2024
This longitudinal study followed 408 participants with COVID-19 chemosensory dysfunction for one year across four major pandemic waves (2020-2023). In a subset of 108 participants, clinical smell and taste tests were conducted and compared with controls without chemosensory dysfunction. Here, we find chronic COVID-19 chemosensory dysfunction is associated with cigarette smoke phantom smells, while environmental allergies and hypertension may predict recovery. We also noted severe subjective and objective distortions in smell and taste during the early pandemic waves (2020-mid 2021), with a resurgence following 2022 infections. Finally, treatments did not resolve chronic chemosensory dysfunction, but clinical assessments confirmed measurable reductions in smell and taste amongst chronic COVID-19 participants. These findings reveal the deep and lasting burden of COVID-19 chemosensory dysfunction, underscoring its profound impact on quality of life.
Interested in learning more about the latest in research and exciting new updates?
Check out the 2024 Newsletter for the Brigham and Women’s Hospital COVID-19 Smell and Taste Dysfunction Study
Featured Publications
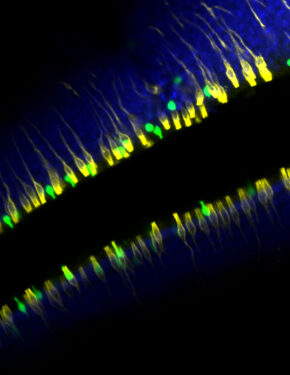
Credit: Alexander Perniss, Ph.D.
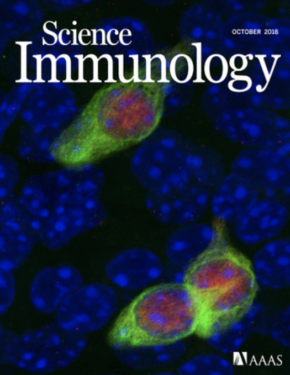
Ualiyeva, Lemire, Wong, & Perniss et al., Science Immunology, 2024
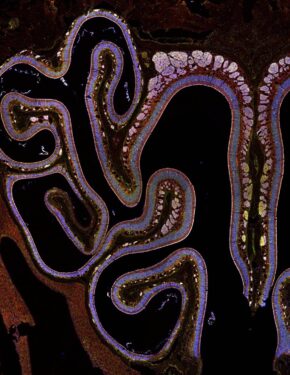
The olfactory neuroepithelium, which plays a key role in odor detection and forms part of the nasal mucosal protective barrier, is composed of various epithelial cells, including microvillous cells (MVCs). Here, we identify a family of MVCs as comprising several tuft cell subtypes, including TRPM5+ tuft cells, solitary chemosensory cells, and glandular DCLK1+ cells, across the olfactory and respiratory epithelia. The investigation reveals that TRPM5+ tuft-MVCs are particularly important in the olfactory epithelium for managing responses to airborne allergens. These cells are essential for the proliferation of olfactory stem cells after allergen exposure. The findings highlight the role of TRPM5+ tuft-MVCs in orchestrating mucosal responses to allergens and underscore the complex functional diversity within the tuft cell lineage.
Ualiyeva et al., Science Immunology, 2021
When tuft cells (a rare type of epithelial cell) sense aeroallergens, they initiate type 2 inflammation, the central feature of asthma and other airway diseases. Tuft cells produce the inflammatory mediators IL-25 and cysteinyl leukotrienes (CysLTs), but the individual and cooperative contributions of these mediators to the immune response remain unknown. Here, we find that IL-25 and CysLTs synergize upon concurrent administration to initiate type 2 inflammation in the lung. Tuft cell-specific deletion of Ltc4s, the enzyme required to generate CysLTs, reduces this effect, revealing the importance of tuft cell-derived CysLTs in the lung type 2 immune response.
In mice, IL-25 and CysLTs proteins from airway #TuftCells cooperate to drive #Allergen inflammation in the lungs, say @BrighamWomens @HarvardMed researchers. https://t.co/VYiUtZAoze pic.twitter.com/6WI5orXQDU
— Science Immunology (@SciImmunology) December 24, 2021
Ualiyeva et al., Science Immunology, 2020
Using an ex vivo and in vivo system, we report that chemosensory airway brush cells (tuft cells) are activated by ATP as well as the aeroallergens Alternaria alternata (a mold allergen) and Dermatophagoides pteronyssinus (house dust mite allergen) for generation of eicosanoids, potent proinflammatory mediators of inflammation and vasodilatation. Alternaria-triggered ATP release and ATP-mediated P2Y2 receptor activation augments the aeroallergen-elicited generation of eicosanoids by brush cells. These findings identify a role for cysteinyl leukotrienes in the primary response to aeroallergens and a potent feed forward loop that amplifies allergen-triggered activation of brush cells.
Bankova et al., Science Immunology, 2018
We identify brush cells, a rare population of chemosensory cells in the lower airways, as the primary cell regulated by CysLT3R in the acute response to the aeroallergen Alternaria alternata. Brush cells expand in the lower airways in response to Alternaria and the house dust mite allergen Dermatophagoides farinae in a CysLT- and CysLT3R -dependent fashion. This expansion is associated with the development of CysLT3R-dependent IL-25-driven lung inflammation, pointing to a role of CysLTs and CysLT3R in both epithelial cell fate and the development of type 2 immunity.
Bankova et al., PNAS, 2016
We identify an allergen-triggered pathway for activation of mast cells and generation of cysteinyl leukotrienes independent of allergen sensitization. First exposure to the airborne mold fungus Alternaria triggers innate mast cell degranulation and generation of cysteinyl leukotrienes in the naïve mouse nasal mucosa. These events lead to mucin release and submucosal vasodilatation mediated through the high-affinity receptor for the stable cysteinyl leukotriene- LTE4.
As a division, we pledge to be anti-racist, to cultivate a culture of inclusion and equity, and to work with courage and purpose to challenge the status quo and to confront inequities and injustices in our world.
Lora Bankova is an Assistant Professor at Harvard Medical School and an Associate Physician at Brigham and Women’s Hospital. Her research focuses on defining the specialized functions of epithelial cells in the olfactory and respiratory mucosa.